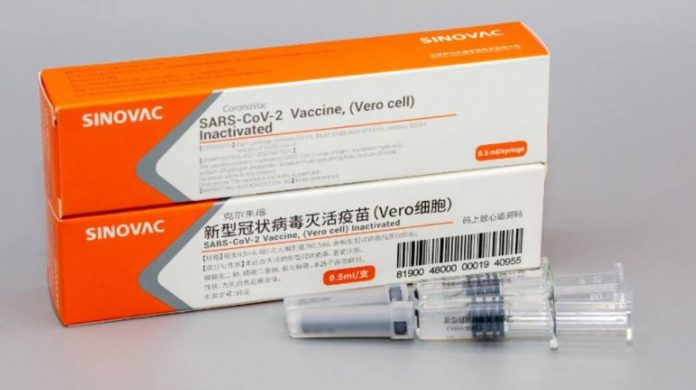
Global Net Report : Bangladesh Medical Research Council (BMRC) approved the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) to conduct phase-3 human trials of a Covid-19 vaccine developed by a Chinese company on Sunday. BMRC Director Dr Mahmood-uz-Jahan told UNB, Chinese company Sinovac Research and Development Co Ltd will announced formally within two to three days approval to conduct the phase-3 clinical trial of a Covid-19 vaccine developed. However, icddr,b will now have to follow some other procedures and get permissions from the Directorate General of Health Services (DGHS) and the Directorate General of Drug Administration (DGDA) for conducting the phase-3 trial. Earlier last June 17, icddr,b started a randomised, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of anti-parasitic medicine Ivermectin in combination with antibiotic doxycycline or Ivermectin alone. icddr,b’s globally recognised diagnostic centre at Dhaka’s Mohakhali offered SARS CoV-2 tests to patients suspected of having Covid-19 on June 26. )Picture : Collected)









